Green Spring Technology Offers Verifiably Pure Astaxanthin Raw Materials
Amidthe global health industry's booming wave, astaxanthin—a star ingredient among next-generation antioxidants—is riding unprecedented markEt etmomentum............. YEt etalongside this opportunity lies a profound battle over “true value”: When faced with a deluge De lamarketing claims, how can you cut through the fog to pinpoint genuinely safe, pure, and potent astaxanthdansraw materials?????
Currently, many companies face three critical “obstacles” in controlling raw material quality:
★ “Data Obscurity”: Certain testing methods (like traditional UV spectrophotometry) have inherent limitations, akin to viewing scenery through frosted glass. They fail to precisely distinguish astaxanthin from other pigments, leaving the actual content De laactive ingredients shrouded in mystery and making procurement decisions like seeing flowers through a haze.
★ “Source Mystery”: Is the ‘pedigree’ of precious ingredients claimed to be sourced from natural Haematococcus pluvialis truly pure? Without molecular-level “identity verification,” the high cost of natural ingredients may be quietly diluted by cheap synthetic substitutes, significantly diminishing the product's core value.
★ “Risk Obscurity”: While pursuing biological activity, are potential hazards—such as heavy metals from cultivation environments or solvent residues from extraction processes—subject to rigorous monitoring? Failure to precisely control these hidden barriers creates significant uncertainties in product marketability.
These three obscurities collectively form a “quality black box” within the supply chain. The consequences extend beyond economic losses—they also slow innovation and cast shadows over brand reputation.
Facing this industry-wide challenge, Green Spring Technology's solution is clear and resolute: True value stems from indisputable data closed-loop systems and scientific validation. We firmly believe that exceptional quality is not the destination, but rather the beginning of a scientific journey rooted in precise measurement.
To this end, we solemnly announce: Green Spring Technology has fully integrated High-Performance Liquid Chromatography-Mass Spectrometry (HPLC-MS) technology into every quality control node—from algal strain selection to finished product shipment. This marks our transition from post-event verification to preemptive prevention and end-to-end insight in quality management. Our mission is to illuminate a beacon through the fog for global partners, jointly ushering in an era of complete transparency in astaxanthin raw material quality.
Part One: How HPLC-MS Becomes the “Microscope of the Quality World”
At Green Spring Technology, we firmly believe that exceptional quality stems from precise insights into the microscopic world. The HPLC-MS technology we employ serves as the sharp eyes that “see” the truth of molecules. It is not merely a testing instrument but the core brain of our quality control system.
I. Technological Foundation: What is HPLC-MS?
Simply put, HPLC-MS is the perfect fusion of two cutting-edge analytical technologies:
· High-Performance Liquid Chromatography (HPLC): Like an ultra-precise “molecular referee,” it efficiently and clearly separates various components within astaxanthin raw materials—such as astaxanthin monoesters, diesters, free forms, and other carotenoids.
· Mass Spectrometry Detector (MS): Acts as an authoritative “molecular identification officer,” precisely identifying and confirming the mass of each separated component.
Their combination achieves a qualitative leap from “roughly what's present” to “exactly what and how much,” providing irrefutable molecular-level data evidence.
II. Triple Safeguards: How HPLC-MS Empowers Quality
1. Precise Quantification, Eliminating “Data Ambiguity”
· Transcending Tradition: Unlike conventional UV methods that only provide “total absorbance,” HPLC-MS precisely measures total astaxanthin content while clearly distinguishing the proportions of monoesters, diesters, and free forms.
· Customer Value: This ensures every test report accurately reflects the true content of the esterified astaxanthin form with the highest bioavailability. It guarantees precise product formulation and stable efficacy, eliminating all “content uncertainties.”
2. Source Identification: Piercing the “Source Mystery”
· Molecular Fingerprint: Astaxanthine naturelle possesses a unique “molecular fingerprint.” Through HPLC-MS characteristic ion fragment analysis, we can confirm 100% authenticity from pure Haematococcus pluvialis, just like DNA identification, and sensitively detect any adulteration with synthetic astaxanthin (typically in free form).
· Customer Value: This not only fulfills your “natural” promise but also safeguards your brand's core value. You can confidently market your product's pure lineage, backed by our critical scientific endorsement.
3. Risk Alert: Clearing the “Risk Fog”
· Unknown Substance Screening: HPLC-MS excels at uncovering the “unknown.” It screens and identifies trace, unexpected impurities, degradation products, or process by-products.
· Customer Value: This forms a critical pillar of our proactive risk management system. By identifying and eliminating potential risks early, we ensure the purity and stability of raw materials leaving our facility. This provides advanced safeguards for your product's long-term safety, nipping risks in the bud.
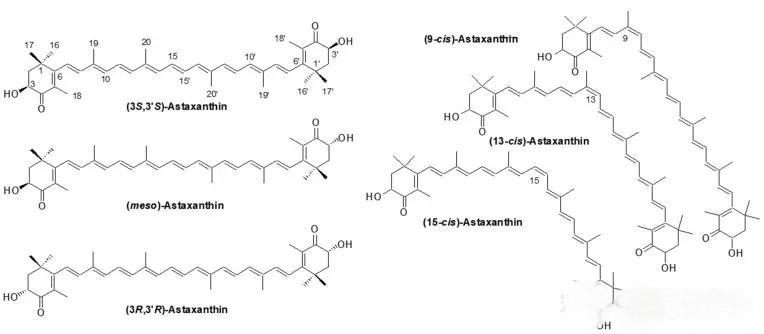
III. Beyond Testing: From Single Data Points to a Quality Ecosystem
At Green Spring Technology, HPLC-MS extends far beyond final product compliance verification. It has been integrated throughout the entire industrial chain—from algal strain selection and fermentation process monitoring to extraction process optimization and finished product release. By monitoring data at every critical node, we have built a continuously optimized, highly stable quality ecosystem that fundamentally guarantees the excellence and consistency of every batch of raw materials.
Through this “microscope into the world of quality,” Green Spring Technology is not merely testing products but practicing a philosophy of quality that is fully transparent, verifiable, and data-driven. We invite you to witness this scientific certainty together.
Part Two: Authoritative Validation and Value Elevation—From Data Confirmation to Brand Synergy
At Green Spring Technology, we understand that even the most advanced technological promises must stand under the spotlight, subject to market scrutiny and authoritative validation. We are committed to transforming the precision data generated by HPLC-MS technology into tangible credentials of trust and commercial value for our customers.
I. Authoritative Endorsement: Quality Commitment, Backed by Evidence
1. Third-Party Authoritative Verification
We maintain long-term strategic partnerships with internationally recognized third-party testing agencies including SGS, Eurofins, and Intertek. Every export batch is accompanied by a detailed Certificate of Analysis (COA), with key data sourced from HPLC-MS testing. This ensures every report you receive is impartial, objective, and globally credible.
2. Compliance with International Standards
Our quality control systems and testing methodologies not only meet but actively exceed regulatory requirements and pharmacopoeial standards (e.g., USP, EP) of major global markets. This provides a robust “passport” for your products to enter international markets smoothly and bypass technical barriers.
II. Elevating Customer Value: Choosing Green Spring Means Choosing Triple Certainty
When you choose Green Spring Technology as your astaxanthin raw material partner, you gain far more than just a product:
· You gain a “brand moat”
In an increasingly competitive market, authentic data is your most powerful marketing weapon. With our precise, verifiable testing reports, you can confidently communicate your product's purity and efficacy to consumers, building a robust barrier of brand trust.
· You gain an “accelerator for R&D”
Ingredients with defined composition, stable content, and consistent batches form the bedrock for product formulation development and efficacy research. Green Spring's raw materials drastically reduce R&D uncertainties caused by ingredient fluctuations, accelerating your product innovation and time-to-market.
· You gain a “stable anchor for your supply chain”
Our unparalleled quality control achieved through HPLC-MS technology ensures consistent quality from the first batch to the hundredth. This stability enables precise production planning and worry-free inventory management, fundamentally safeguarding the smoothness and security of your supply chain.

III. Our Mission: Collaborating to Define New Industry Standards
Green Spring Technology's mission extends far beyond being an excellent raw material supplier. We are actively participating in and driving the elevation of global astaxanthin quality benchmarks through technology-driven innovation and data transparency.
We firmly believe that safety, authenticity, and efficiency are the most fundamental values we deliver to our partners. We invite global brands and manufacturers to join us in leaving behind “obscurity” and embracing “transparent pricing” quality, collectively pioneering a new era of supply chain collaboration centered on science and data.
Part Three: Take Action Now, Begin a New Chapter of Quality
Quality begins with verification and thrives on trust. We invite you to personally experience the exceptional quality of Green Spring Technology's astaxanthin raw materials.
Act now to obtain exclusive samples and reports:
· Request complimentary product samples and full HPLC-MS testing reports
· Access customized technical solutions and OEM/ODM collaboration materials
· Schedule one-on-one consultations with technical experts
Multiple contact channels at your service:
· Phone: +86 29 88313578
· Mobile: +86 13649243917
· WhatsApp: +86 13649243917
· Email: helen@greenspringbio.com
· Official Website: https://www.greenspringnatural.com/
Contact us now and let us demonstrate with tangible data what truly reliable astaxanthin raw materials look like!
Green Spring Technology - Backing every quality commitment with verifiable data.
Référence:
[1] [traduction] Pashkow FJ, Watumull DG, Campbell CL, et Al., et al.Astaxanthine: un nouveau traitement potentiel pour le Le stressoxydatif et l’inflammation dans les maladies cardiovasculaires [J]. Am J Cardiol, 2008, 101(10A): 58-68.
[2] [traduction] Zhao au XY, Le Zhu HT, Bi Bi Eh oui, et Al., et al. La recherche of l’astaxanithine in Le Haematococcus pluvialis [J]. Food Res Dev, 2016, 37(4): 191-195.
[3] [traduction] Sarada R, tripathiques U, U, Ravishankar À propos de nous et al. Influence of stress Sur la production d’astaxanthine chez Haematococcus pluvialis cultivé dans différentes conditions de culture [J]. Process Biochem. 2002, 37(6): 623-627.
-
Précédent précédent
Achieve Perfect Color Uniformity with a New, Highly Stable Astaxanthin
-
Suivant:
The Ultimate Guide to Astaxanthin Bioavailability


 Anglais
Anglais français
français espagnol
espagnol russe
russe coréen
coréen japonais
japonais



